Featured
- Get link
- X
- Other Apps
Specific Latent Heat Calculations
Specific Latent Heat Calculations. The specific latent heat of a material can be found by measuring the energy needed to change the state of the material. The increase internal energy during the time when the temperature remains constant is the energy required to melt the material.
Apply heat to bronze that is below 880 ° c. The amount of heat energy absorbed or released for a phase change is known as latent heat. During the calculation of latent heat a formula can be quite handy and makes your job much simple.
Related Calculators Acentric Factor Braking Torque Motor Calculate Heat Transferred To System.
Apply heat to aluminum that is less than 620 ° c. Apply heat to bronze that is below 880 ° c. The formula for latent heat:
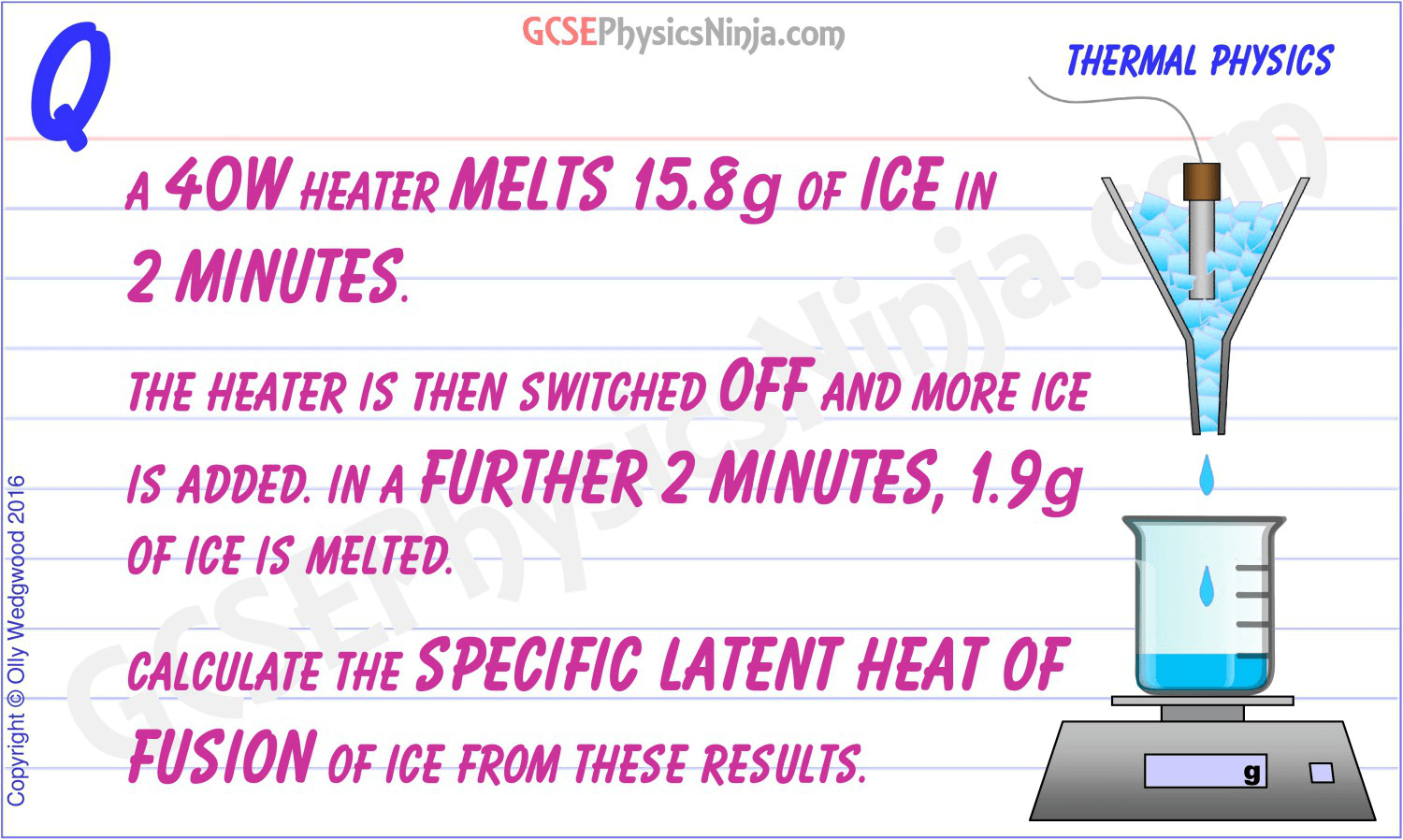
A Method For Measuring The Specific Latent Heat Of Fusion Of Water.
Table of specific latent heats. Using these values, the heat of condensation q c to be released for a given mass m c to be condensed can be determined with the following formula: Energy in heat (q) kj.
Insert The Amount Of Energy Supplied As A Positive Value.
In the case of condensation, again. Q is the heat absorbed or released based on the direction of transition and its units are kj. In addition, we will study the effectiveness of different calorimeters.
Determine The Latent Heat Of 5 Kg Substance If The Amount Of Heat For A Phase Change Is 300 K.cal.
Latent heat formula is q = m * l. There are three types of specific latent heat. L = q / m.
This Can Be Used To Calculate The Specific Latent Heat Of Fusion.
For change in state use: Where m is the mass of the body and its units are kg. Here, l takes the place of the.
Popular Posts
Calculate The Equilibrium Potential For Na+ At 20C
- Get link
- X
- Other Apps
Comments
Post a Comment